Much like fluorescence microscopy, stimulated Raman scattering (SRS) gain microscopy is an optical imaging method capable of generating high-resolution maps of biological tissues1. The contrast in SRS images derives from the characteristic vibrations of the sample’s molecules, which enable tissue imaging without the need to label samples with fluorescent dyes. SRS is gaining ground as a biomedical imaging tool, but the minimum molecular concentrations that it can detect are higher than those that can be detected using fluorescence microscopy, thus limiting its scope. Finding ways to fundamentally improve the detection sensitivity of this technique has been challenging. Writing in Nature, Casacio et al.2 describe an approach for boosting sensitivity through quantum-enhanced suppression of noise in the SRS signal.
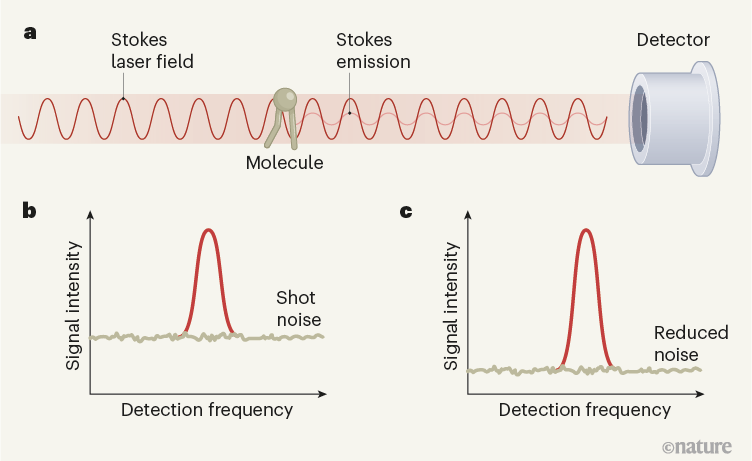
SRS is based on a phenomenon called the Raman effect, which involves two photons: one known as the pump photon (of frequency ω1), which interacts with a molecule; and another called the Stokes photon (of a lower frequency, ω2), which is radiated by the molecule in response to its interaction with the pump photon. The frequency difference (ω1 ω2) corresponds to the frequency of a particular vibrational mode of the molecule. Measurements of the wavelength of the Stokes photons can therefore be used to identify the molecule on the basis of its vibrational behaviour. However, the signal produced by the Raman effect is weak, giving rise to a long integration time (the period needed to collect a signal), which prevents it from being used for tissue imaging.
SRS cleverly overcomes the weakness of the Raman signal. Unlike the linear Raman effect described above, which uses one laser beam, SRS involves illuminating the sample with two laser-light fields: a pump field with frequency ω1 and a Stokes field with frequency ω2. In SRS, the sample produces a stronger signal field than in the linear effect. Moreover, because the signal field is produced in phase with the Stokes field, constructive interference occurs, greatly boosting the signal from the sample (Fig. 1). The amplified signal is therefore much stronger than the weak molecular signal produced by the linear effect.
The enhanced Raman signal enables fast imaging: in some cases, SRS imaging can be up to one million times faster than that achieved using linear Raman microscopy3. However, SRS signal detection is more complicated than in the linear effect, because the enhanced signal has to be distinguished from the towering background of the Stokes laser field. The ratio of the SRS signal to the laser background is typically of the order of 10–5–10–6, necessitating special amplification techniques to retrieve the desired signal.
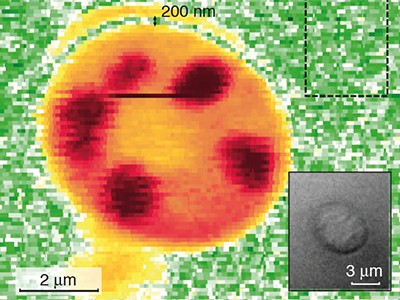
Any fluctuation in the background laser intensity makes it even more difficult to detect the SRS contribution4. Even when all other sources of noise — such as electronic noise from the detector — have been suppressed, random fluctuations in the laser background still impose a limit on the detection sensitivity. These fluctuations are called shot noise, and arise from the fact that the laser beam consists of discrete units of light (photons). Shot noise imposes a lower limit, known as the noise floor, on the amount of noise in SRS signals. The success of modern SRS microscopy has relied on the ability to reduce laser noise down to this limit. Nevertheless, weak signals remain buried beneath the noise floor, restricting the sensitivity of SRS microscopy and making it difficult to detect molecular targets at concentrations below 1 millimole per litre.
In principle, the SRS signal-to-noise ratio can be improved by increasing the number of photons in the laser beams. But there is a limit to the illumination dose that biological samples can tolerate without damage5,6. SRS imaging studies usually operate at laser intensities just below the damage threshold, leaving little margin for improvement. And simple measures that increase the SRS signal strength relative to the noise floor, without introducing other complications, are hard to come by.
Casacio et al. improve SRS sensitivity in a radically different way. Instead of raising the signal, they lower the background noise, allowing smaller SRS signals to peak above the noise floor, like rocks exposed on a beach at low tide. To realize this idea, the detection limit set by shot noise has to be overcome, which can be accomplished when there are quantum correlations between the photons that make up the applied light beam7,8. The use of such ‘non-classical’ light has been particularly successful in reducing shot noise in interferometry experiments, such as those used to detect gravitational waves9. The SRS process also depends on the interference between two light fields, suggesting that the use of non-classical light should translate well to SRS imaging.
The authors used a laser beam that is prepared in a ‘squeezed-amplitude state’. In this quantum state, the Stokes photons are no longer fully independent — which means that fluctuations in the number of photons in the beam no longer follow the statistical distribution observed in classical laser beams. By lowering the noise floor below the shot-noise limit, the researchers obtained a 35% improvement in the signal-to-noise ratio of SRS imaging, pushing down the minimum molecular concentration that could be detected by 14%. This improvement was achieved without increasing the intensity of the laser beams, thereby preserving the integrity of biological samples.
It should be noted that the currently reported improvements are modest, and the resulting performance is still below that of state-of-the-art SRS systems. Impressive noise reduction has been attained in other techniques by squeezing narrow-band laser light (see ref. 9, for example), but it might prove challenging to achieve similar reductions using the broadband laser beams that are needed for SRS. Moreover, the process of ‘squeezing’ light to produce correlations between photons adds an extra step to the already-complicated SRS imaging technique, and might deter users from adopting this noise-reduction approach.
Nevertheless, Casacio and colleagues’ work underlines the exciting possibilities of using quantum light in optical imaging techniques. Despite the challenges ahead, quantum light is likely to transform SRS microscopy for the better.
"light" - Google News
June 09, 2021 at 10:21PM
https://ift.tt/3cwBrad
Squeezed light improves sensitivity of microscopy technique - Nature.com
"light" - Google News
https://ift.tt/2Wm8QLw
https://ift.tt/2Stbv5k
Bagikan Berita Ini














0 Response to "Squeezed light improves sensitivity of microscopy technique - Nature.com"
Post a Comment